WHAT ARE CLINICAL TRIALS
When it comes to proving whether an intervention or clinical solution will achieve what it did in the laboratory (sometimes called “preclinical” setting), in human beings (often referred to the “clinical” setting), the final, common pathway is clinical trials. The intervention could include medications, behavioural intervention, medical device, surgical procedure or other experimental therapies.
Understanding clinical trials
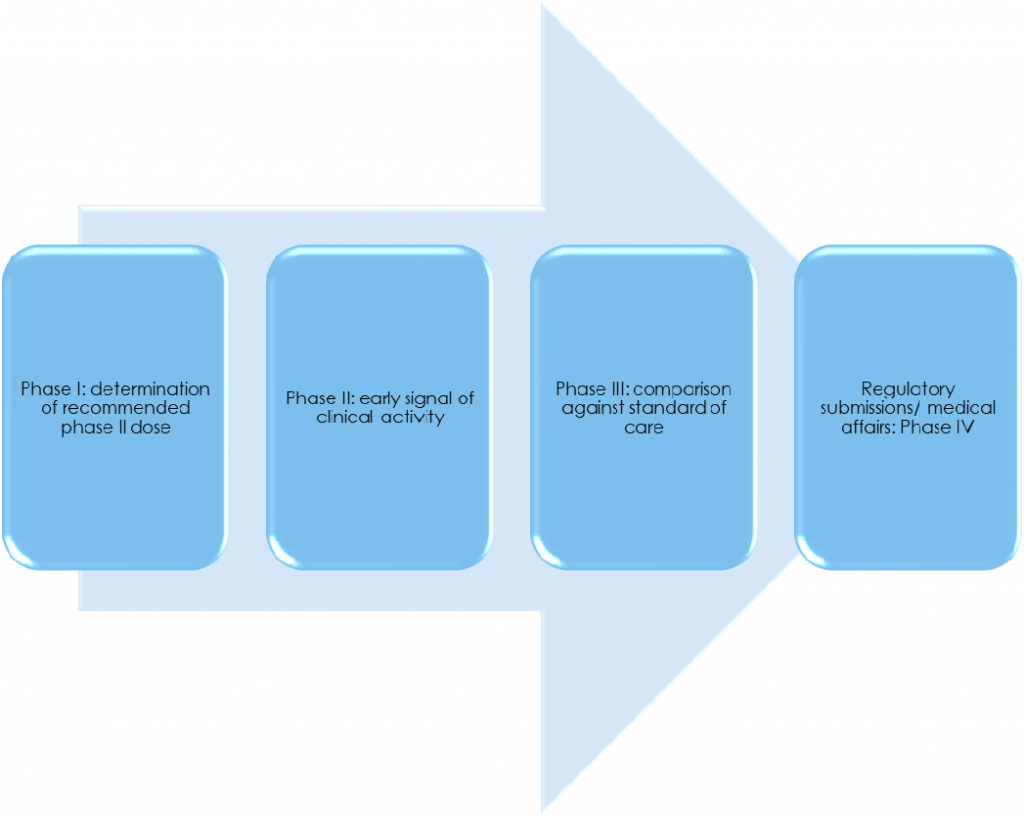
Any clinical solution must first prove itself to be safe and effective in the preclinical before entering clinical research phase. There are four phases of clinical trials:
Phase I
Phase I is the very first time a novel intervention and/or novel combination of interventions is used for the first time in human beings. Generally, the main aim of a phase I study is safety, although this is changing.
Phase II
In phase II, the main aims are usually to evaluate efficacy and to look for additional safety signals.
Phase III
Phase III clinical trials usually involve comparisons against current standard of care, to “place” the clinical solution in the spectrum of current treatment landscape of the disease area of interest. Treatment-related adverse events are monitored continuously throughout the clinical research phase of drug development to collect information and allow the intervention to be used safely in future, broader patient group.
Phase IV
Phase IV clinical trials may be conducted following regulatory approval/post-marketing to look for additional safety signal.
Clinical trials CRO
A good clinical trials CRO will be able to provide a significant advice to the study sponsor on how best to run the study so it is efficient and also effective.
Obatica is a specialised clinical trials CRO that focuses on providing study design, medical monitoring, pharmacovigilance, and medical writing services. Contact us to discuss your study.
An early investment in a high-quality drug development plan is a great way to set up your clinical solution for eventual regulatory success.
